01
иғҢжҷҜ
й”ӮзҰ»еӯҗеҠЁеҠӣз”өжұ еңЁеҢ–жҲҗгҖҒиҝҮе……/иҝҮж”ҫгҖҒзғӯеӨұжҺ§гҖҒеҫӘзҺҜиҝҮзЁӢйғҪдјҡжңүж°”дҪ“еҸ‘з”ҹгҖӮеҢ–жҲҗйҳ¶ж®өпјҢй”ӮзҰ»еӯҗз”өжұ еҶ…йғЁз”ұдәҺеӣәжҖҒз”өи§ЈиҙЁз•ҢйқўиҶң(Solid Electrolyte Interface, SEI) зҡ„еҪўжҲҗдјҡдә§з”ҹдёҖе®ҡйҮҸзҡ„ж°”дҪ“гҖӮж°”дҪ“зҡ„еҗ«йҮҸгҖҒж°”дҪ“з§Қзұ»е’Ңж°”дҪ“еҸҳеҢ–зҺҮзӯүж–№йқўеҸҜд»ҘеҸҚжҳ SEIеҪўжҲҗзҡ„иҙЁйҮҸе’ҢзЁӢеәҰпјҢиҝӣиҖҢеҸҚжҳ з”өжұ еҢ–жҲҗзҡ„еҘҪеқҸгҖӮиҝҮе……/иҝҮж”ҫж—¶пјҢз”өи§Јж¶ІеңЁжӯЈиҙҹжһҒиў«ж°§еҢ–иҝҳеҺҹиҖҢеҲҶи§ЈпјҢжӯЈжһҒжқҗж–ҷж°§жһҗеҮәзӯүдә§з”ҹеӨ§йҮҸж°”дҪ“гҖӮиҝҮе……/иҝҮж”ҫз”ҡиҮідјҡиҝӣдёҖжӯҘеј•еҸ‘зғӯеӨұжҺ§пјҢеҸ‘з”ҹй“ҫејҸеҸҚеә”пјҢдә§з”ҹеӨ§йҮҸж°”дҪ“гҖӮз”өжұ еңЁжӯЈеёёиҝҗиЎҢиҝҮзЁӢдёӯпјҢз”өжһҒиЎЁйқўеүҜеҸҚеә”дёҚж–ӯиҝӣиЎҢпјҢд№ҹдјҡзј“ж…ўең°дә§з”ҹж°”дҪ“гҖӮеңЁеҠЈеҢ–дёҘйҮҚзҡ„жғ…еҶөдёӢпјҢз”өжұ еҸ‘з”ҹйј“еҢ…пјҢеҪұе“ҚеҲ°з”өжұ зҡ„е®үе…Ёе’ҢжҖ§иғҪгҖӮж°”дҪ“жҲҗеҲҶе’Ңеҗ«йҮҸзӣҙжҺҘеҸҚжҳ дәҶз”өжұ еҶ…йғЁеүҜеҸҚеә”еҸ‘з”ҹзҡ„зЁӢеәҰпјҢдёҺз”өжұ зҡ„еҒҘеә·е’Ңе®үе…ЁзҠ¶жҖҒеҜҶеҲҮзӣёе…ігҖӮз ”з©¶з”өжұ еҗ„йҳ¶ж®өж°”дҪ“дҝЎеҸ·пјҢе®ҡйҮҸиЎЁеҫҒеҸҠеҲҶжһҗз”өжұ дә§ж°”жғ…еҶөиҮіе…ійҮҚиҰҒгҖӮеҪ“еүҚй”ӮзҰ»еӯҗз”өжұ ж°”дҪ“зҡ„иЎЁеҫҒж–№жі•еҸҜд»ҘеҲҶдёәйқһеҺҹдҪҚе’ҢеҺҹдҪҚиЎЁеҫҒдёӨз§Қж–№ејҸгҖӮ
02
ж°”дҪ“жҲҗеҲҶиЎЁеҫҒж–№жі•
2.1 ж°”дҪ“жҲҗеҲҶйқһеҺҹдҪҚиЎЁеҫҒж–№жі•
зӣ®еүҚпјҢз”өжұ ж°”дҪ“жҲҗеҲҶзҡ„ж–№жі•еӨҡеҖҹеҠ©йқһеҺҹдҪҚиЎЁеҫҒзҡ„жқҗж–ҷеӯҰеҲҶжһҗжүӢж®өпјҢеҰӮеӣҫ1жүҖзӨәпјҢеҢ…еҗ«ж°”зӣёиүІи°ұиҙЁи°ұжі•(Gas Chromatography-Mass Spectrometry, GC-MS)[1]пјҢеӮ…йҮҢеҸ¶еҸҳжҚўзәўеӨ–е…үи°ұжі•(Fourier Transform-Infrared Spectroscopy, FT-IR)[2,3]пјҢж ёзЈҒе…ұжҢҜе…үи°ұ(Nuclear Magnetic Resonance, NMR)[4-7]гҖӮ
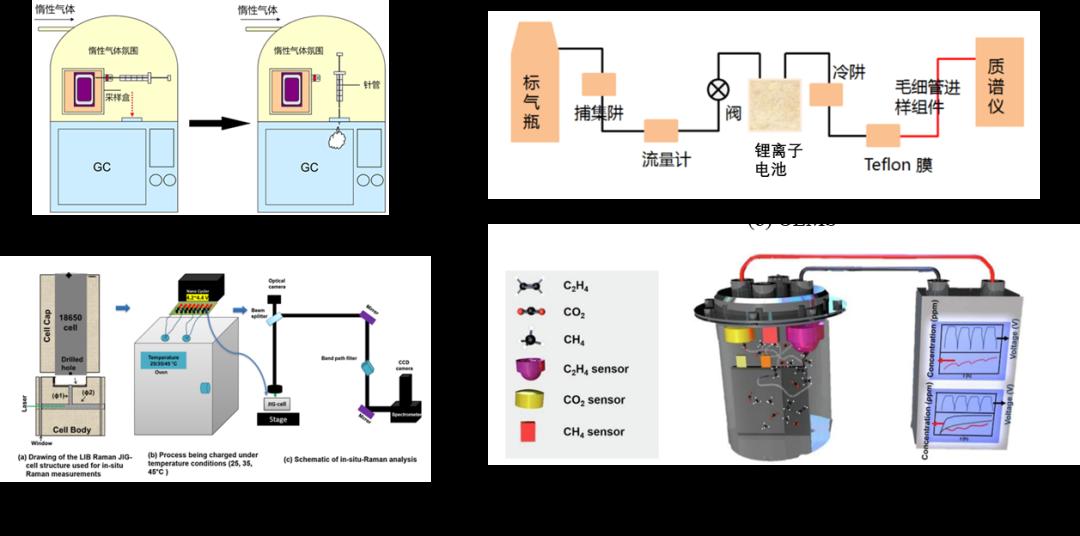
еӣҫ 1 ж°”дҪ“жҲҗеҲҶзӣ‘жөӢж–№жі•
W. Kongзӯүдәә[8] е°ҶLiCoO2гҖҒLiMn2O4е’ҢLiFePO4дёүз§ҚжӯЈжһҒжқҗж–ҷзҡ„18650й”ӮзҰ»еӯҗз”өжұ жӯЈеёёе……з”өе’ҢиҝҮе……иҮі4.5 Vе’Ң5.0 VпјҢ然еҗҺз”ЁжіЁе°„еҷЁж”¶йӣҶз”өжұ еҶ…йғЁзҡ„ж°”дҪ“并йҮҮз”ЁGC-MSжөӢйҮҸдәҶж°”дҪ“жҲҗеҲҶпјҢеҰӮеӣҫ1(a)жүҖзӨәгҖӮз ”з©¶еҸ‘зҺ°пјҢеңЁжӯЈеёёе……з”өжқЎд»¶дёӢпјҢж°”дҪ“еҸ‘з”ҹиЎҢдёәдёҺжӯЈжһҒжқҗж–ҷзұ»еһӢж— е…ігҖӮеңЁиҝҮе……жқЎд»¶дёӢпјҢйҳҙжһҒжқҗж–ҷзҡ„ж°§еҢ–иғҪеҠӣеҜ№ж°”дҪ“з§Қзұ»е’Ңж•°йҮҸжңүжҳҫи‘—еҪұе“ҚгҖӮFredrik Larssonзӯүдәә[9]з”Ё7з§ҚдёҚеҗҢзұ»еһӢй”ӮзҰ»еӯҗз”өжұ иҝӣиЎҢдәҶзҮғзғ§е®һйӘҢпјҢз”ЁFIRTе®ҡйҮҸжөӢйҮҸдәҶз”өжұ зҮғзғ§жүҖйҮҠж”ҫзҡ„ж°”дҪ“пјҢз»“жһңиЎЁжҳҺзҮғзғ§дјҡдә§з”ҹеӨ§йҮҸзҡ„ж°ҹеҢ–ж°ў(HF)е’Ңе°‘йҮҸзҡ„ж°ҹеҢ–зЈ·(POF3)жңүжҜ’ж°”дҪ“гҖӮNina Laszczynskiзӯүдәә[10]еҜ№NCM811з”өжұ й«ҳеҺӢдёӢз”өи§ЈиҙЁеҲҶи§Јжғ…еҶөиҝӣиЎҢдәҶз ”з©¶пјҢдҪҝз”ЁNMRзӯүеӨҡз§Қж–№жі•жөӢйҮҸдәҶз”өи§ЈиҙЁеҲҶи§Јзҡ„ж°”дҪ“жҲҗеҲҶпјҢз ”з©¶еҸ‘зҺ°еҪ“жҲӘжӯўз”өеҺӢд»Һ4.2 VеўһеҠ еҲ°4.6 Vж—¶пјҢO2е’ҢCO2зҡ„йҮҠж”ҫйҮҸйҡҸд№ӢеўһеҠ гҖӮйқһеҺҹдҪҚзҡ„ж–№жі•дёҚиғҪжЈҖжөӢе……ж”ҫз”өеҫӘзҺҜдёӯж—¶й—ҙеҲҶиҫЁзҺҮдёҠз”өжұ дә§ж°”зҡ„жғ…еҶөпјҢйңҖиҰҒеңЁз”өжұ е®һйӘҢз»“жқҹеҗҺеңЁйқһеӨ§ж°”жҡҙйңІжқЎд»¶дёӢйҖҡиҝҮз ҙзҺҜжҖ§зҡ„ж–№ејҸиҺ·еҸ–з”өжұ еҶ…йғЁж°”дҪ“иҝӣиЎҢе®ҡйҮҸжөӢйҮҸгҖӮ
2.2 ж°”дҪ“жҲҗеҲҶеҺҹдҪҚиЎЁеҫҒж–№жі•
ж°”дҪ“жҲҗеҲҶеҺҹдҪҚе®һж—¶иЎЁеҫҒзҡ„ж–№жі•йҮҮз”ЁеңЁзәҝ/еҫ®еҲҶз”өеҢ–еӯҰиҙЁи°ұжҠҖжңҜ(Online/Differential Electrochemical Mass Spectrometry, O/DEMS)[1,11,12]пјҢеҺҹдҪҚжӢүжӣје…үи°ұ(In-situ Raman Spectra, IRS)[13,14]е’ҢйқһиүІж•ЈзәўеӨ–ж°”дҪ“дј ж„ҹеҷЁ(Nondispersive Infrared Gas Sensors, NDIR)[7,15,16]зӣ‘жөӢж°”дҪ“жҲҗеҲҶйҡҸз”өдҪҚе’Ңж—¶й—ҙжј”еҸҳжғ…еҶөпјҢеңЁз ”究йўҶеҹҹеҸ—еҲ°е№ҝжіӣе…іжіЁгҖӮO/DEMSжҳҜе°Ҷз”өеҢ–еӯҰеҸҚеә”жұ дёҺиҙЁи°ұд»ӘиҒ”з”ЁпјҢеҸҜд»Ҙе®һж—¶жЈҖжөӢз”өеҢ–еӯҰеҸҚеә”з•Ңйқўж¶ҲиҖ—жҲ–дә§з”ҹзҡ„ж°”дҪ“е’ҢжҢҘеҸ‘жҖ§дёӯй—ҙдә§зү©еҸҠжңҖз»Ҳдә§зү©пјҢ并иҝӣиЎҢе®ҡжҖ§е’Ңе®ҡйҮҸеҲҶжһҗгҖӮN. Р•. Galushkinзӯүдәә[17]дҪҝз”ЁO/DEMSзӣ‘жөӢдәҶNMC111з”өжұ еңЁдёҚеҗҢдёҠжҲӘжӯўз”өеҺӢе’Ңжё©еәҰжқЎд»¶дёӢеҫӘзҺҜиҝҮзЁӢдёӯжӯЈиҙҹжһҒдә§ж°”зҡ„жғ…еҶөпјҢеҰӮеӣҫ1(b)гҖӮз»“жһңиЎЁжҳҺз”өи§ЈиҙЁеҲҶи§Јдјҡдә§з”ҹCOе’ҢH2ж°”дҪ“пјҢиҖҢCO2еҸӘеңЁжӯЈжһҒз”ҹжҲҗжҳҜжӯЈжһҒеҺҹеӯҗжҷ¶ж јйҮҠж”ҫзҡ„O2дёҺжӯЈжһҒйҷ„иҝ‘COпјҲз”өи§Јж¶ІеҲҶи§ЈпјүеҸҚеә”зҡ„дә§зү©гҖӮиҷҪ然O/DEMSеҸҜд»ҘеҺҹдҪҚжөӢйҮҸж°”дҪ“жҲҗеҲҶпјҢдҪҶжҳҜиҜҘж–№жі•йңҖиҰҒиҪҪж°”иЈ…зҪ®е°Ҷж°”дҪ“иҫ“йҖҒеҲ°иҙЁи°ұд»ӘпјҢиҝҷе°Ҷеј•иө·з”өи§ЈиҙЁжҢҘеҸ‘пјҢиҝӣиҖҢеҪұе“Қз”өжұ жӯЈеёёиҝҗиЎҢгҖӮByambasuren Gerelt-Odзӯүдәә[18]ејҖеҸ‘дәҶз”ЁдәҺз”өжұ еҲҶжһҗзҡ„IRSеҲҶжһҗзі»з»ҹпјҢеҰӮеӣҫ1(c)жүҖзӨәпјҢе…¶еңЁе•Ҷдёҡз”өжұ дёҠе®үиЈ…зҡ„зҺ»з’ғзӘ—иҝӣиЎҢжҝҖе…үж•Је°„пјҢеӣ жӯӨеҸҜд»Ҙж— е№Іжү°ең°и·ҹиёӘз”өжұ еҶ…йғЁз”өеҢ–еӯҰеҸҚеә”гҖӮз ”з©¶з»“жһңиЎЁжҳҺж»Ўз”өзҠ¶жҖҒзҡ„18650з”өжұ еңЁ25-45в„ғзҺҜеўғдёӢеӯҳеӮЁпјҢдјҡйҖҗжёҗдә§з”ҹH2, CH4, CO2е’ҢCOеӣӣз§Қдё»иҰҒж°”дҪ“пјҢиҝҮйҮҸзҡ„H2дҪҝз”өжұ еӯҳеңЁе®үе…ЁйҡҗжӮЈгҖӮSiqi Lyuзӯүдәә[19]ејҖеҸ‘дәҶеҹәдәҺNDIRзҡ„ж°”дҪ“жҲҗеҲҶзӣ‘жөӢиЈ…зҪ®пјҢе°Ҷдёүз§ҚNDIRзҡ„CO2гҖҒCH4е’ҢC2H4ж°”дҪ“дј ж„ҹеҷЁе’ҢејҖеҸЈзҡ„е•Ҷдёҡз”өжұ е…ұеҗҢж”ҫеңЁеҜҶе°ҒзҪҗдёӯеҰӮеӣҫ1(d)жүҖзӨәпјҢзӣ‘жөӢз”өжұ иҝҗиЎҢж—¶дёүз§Қж°”дҪ“зҡ„жј”еҢ–жғ…еҶөгҖӮз»“жһңиЎЁжҳҺпјҢй«ҳз”өеҺӢдјҡеҜјиҮҙCO2з”ҹжҲҗйҮҸеўһеҠ пјҢиҖҢCH4е’ҢC2H4зҡ„з”ҹжҲҗйҮҸеҜ№жё©еәҰжӣҙж•Ҹж„ҹгҖӮе°Ҫз®ЎIRSе’ҢNDIRд»ҘдёҠдёӨз§Қж–№жі•еҸҜд»Ҙе®һж—¶зӣ‘жөӢе•Ҷдёҡз”өжұ еҶ…йғЁж°”дҪ“жҲҗеҲҶзҡ„жј”еҢ–пјҢдҪҶжҳҜйғҪйңҖиҰҒеҜ№з”өжұ иҝӣиЎҢиҫғеӨ§и§„жЁЎзҡ„ж”№йҖ е’Ңз ҙеқҸпјҢ并且йңҖиҰҒиҝһжҺҘеӨ§еһӢзҡ„ж°”дҪ“и§Јжһҗд»ӘеҷЁпјҢеӨҡз”ЁдәҺзҹӯжңҹдә§ж°”жңәзҗҶи§ЈжһҗгҖӮ
2.3 ж°”дҪ“жҲҗеҲҶеҺҹдҪҚйҮҮйӣҶж–№жі•
ж°”дҪ“еҺҹдҪҚйҮҮйӣҶж–№жі•жҳҜйҖҡиҝҮеҜ№з”өжұ еЈідҪ“иҝӣиЎҢи®ҫи®ЎпјҢеңЁз”өжұ еЈідҪ“еўһеҠ еҸ–ж°”еҸЈпјҢеңЁдёҚеҪұе“Қз”өжұ зҡ„еҠЁжҖҒе·ҘдҪңиҝҮзЁӢзҡ„еүҚжҸҗдёӢпјҢе®һзҺ°еӨҡж¬ЎеҸ–ж°”еҸҠжЈҖжөӢеҲҶжһҗпјҢиҝӣиҖҢе®һзҺ°иҝһз»ӯзӣ‘жөӢж°”дҪ“жҲҗеҲҶзҡ„жј”еҸҳгҖӮзҺӢз»ҘеҶӣзӯүдәә[20]и®ҫи®ЎдәҶдёҖдёӘеҺҹдҪҚж°”дҪ“зӣ‘жөӢиЈ…зҪ®пјҢе…¶дёӯз”өжұ еҶ…йғЁйҖҡиҝҮеҜјз®ЎиҝһжҺҘеӣӣйҖҡйҳҖпјҢеӣӣйҖҡйҳҖдёҺеҺӢеҠӣдј ж„ҹеҷЁгҖҒж°”дҪ“йҮҮж ·еҸЈгҖҒзңҹз©әйҳҖиҝһжҺҘпјҢеҸҜйҡҸж—¶и®°еҪ•з”өжұ еҶ…йғЁеҺӢеҠӣпјҢд№ҹеҸҜд»ҘйҖҡиҝҮж°”еҜҶй’ҲйҡҸж—¶йҮҮеҸ–ж°”дҪ“ж ·е“ҒпјҢеҲҶжһҗж°”дҪ“з»„еҲҶпјҢеҰӮеӣҫ 2(a)жүҖзӨәгҖӮеҹәдәҺжӯӨж–№жі•иҜҘеӯҰиҖ…з ”з©¶дәҶй’ӣй…ёй”Ӯз”өжұ еңЁ55в„ғеҫӘзҺҜиҝҮзЁӢе’Ң55в„ғжҗҒзҪ®е·ҘеҶөдёӢеҶ…йғЁеҺӢеҠӣгҖҒиғҖж°”дҪ“з§ҜгҖҒд»ҘеҸҠеҗ„з»„еҲҶж°”дҪ“еҗ«йҮҸзҡ„еҸҳеҢ–规еҫӢпјҢ并жҺЁеҜјдәҶеҸҜиғҪзҡ„дә§ж°”еҸҚеә”гҖӮJan-Patrick Schmiegelзӯүдәә[21]и®ҫи®ЎдәҶеёҰж°”дҪ“еҸ–ж ·еҸЈзҡ„иҪҜеҢ…й”ӮзҰ»еӯҗз”өжұ пјҢе…¶дёӯеҚ•еҗ‘ж°”дҪ“еҸ–ж ·еҸЈз”ұйІҒе°”жҺҘеҸЈгҖҒGCиҝӣж°”еһ«е’ҢGCйҮҮж ·з“¶зӣ–з»„жҲҗ并йҖҡиҝҮPPеҜјз®ЎиҝһжҺҘз”өжұ еҶ…йғЁпјҢеҰӮеӣҫ 2(b)жүҖзӨәгҖӮиҜҘз ”з©¶дәәе‘ҳйҖҡиҝҮеҚ•еҗ‘еҸ–ж ·еҸЈеӨҡж¬ЎеҸ–ж°”з ”з©¶дәҶеҚ•ж¬Ўе……ж”ҫз”өеҫӘзҺҜй—ҙж°”дҪ“з»„еҲҶзҡ„жј”еҸҳгҖӮ
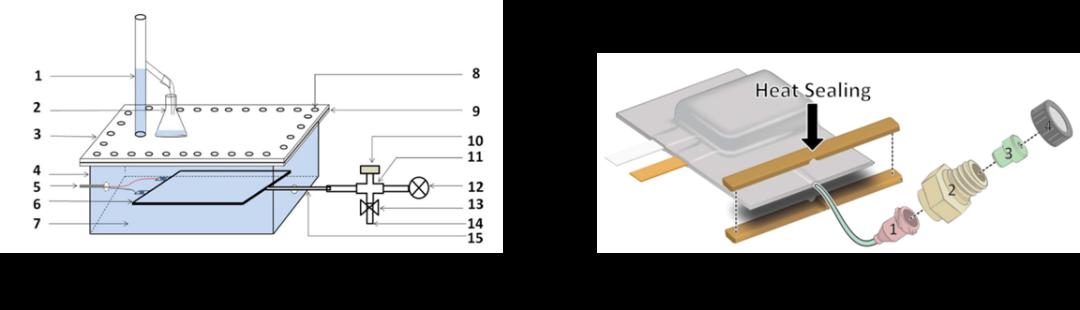
еӣҫ 2ж°”дҪ“жҲҗеҲҶеҺҹдҪҚйҮҮйӣҶж–№жі•
03
жҖ»з»“
еҪ“еүҚж°”дҪ“жҲҗеҲҶиЎЁеҫҒж–№жі•иҫғдёәдё°еҜҢпјҢдҪҶжҳҜеӨ§йғҪйңҖиҰҒдҫқиө–еӨ§еһӢдё“дёҡзҡ„еҲҶжһҗи®ҫеӨҮпјҢйҡҫд»Ҙе®һзҺ°еӮЁиғҪжҲ–иҖ…иҪҰиҪҪеңәжҷҜз”өжұ еҶ…йғЁж°”дҪ“зҡ„е®һж—¶жЈҖжөӢгҖӮжңӘжқҘпјҢй•ҝеҜҝе‘Ҫзҡ„MEMSж°”дҪ“дј ж„ҹеҷЁгҖҒе…үзәӨж°”дҪ“дј ж„ҹеҷЁзӯүеҫ®еһӢдј ж„ҹеҷЁжңүжңӣдёҺеӨ§е®№йҮҸз”өжұ еҚ•дҪ“иҝӣиЎҢйӣҶжҲҗпјҢе®һзҺ°з”өжұ еҶ…йғЁж°”дҪ“зҡ„е®һж—¶жЈҖжөӢпјҢиҝӣиҖҢдёәй”ӮзҰ»еӯҗз”өжұ е®үе…Ёз®ЎжҺ§гҖҒеҒҘеә·з®ЎзҗҶжҸҗдҫӣж–°зҡ„ж–№жі•гҖӮ
04
еҸӮиҖғж–ҮзҢ®
[1]Li, Z., Yao, N., Yu, L., Yao, Y.-X. et al., "Inhibiting gas generation to achieve ultralong-lifespan lithium-ion batteries at low temperatures." Matter 2023, doi: 10.1016/j.matt.2023.04.012.
[2]Bertilsson, S., Larsson, F., Furlani, M., Albinsson, I. et al., "Lithium-ion battery electrolyte emissions analyzed by coupled thermogravimetric/Fourier-transform infrared spectroscopy." Journal of Power Sources 365:446-455, 2017, doi: 10.1016/j.jpowsour. 2017.08.082.
[3]Berkes, B. B., Schiele, A., Sommer, H., Brezesinski, T. et al., "On the gassing behavior of lithium-ion batteries with NCM523 cathodes." Journal of Solid State Electrochemistry 20 (11):2961-2967, 2016, doi: 10.1007/s10008-016-3362-9.
[4]Blanc, F., Leskes, M., and Grey, C. P., "In Situ Solid-State NMR Spectroscopy of Electrochemical Cells: Batteries, Supercapacitors, and Fuel Cells." Accounts of Chemical Research 46 (9):1952-1963, 2013, doi: 10.1021/ar400022u.
[5]Handel, P., Fauler, G., Kapper, K., Schmuck, M. et al., "Thermal aging of electrolytes used in lithium-ion batteries вҖ“ An investigation of the impact of protic impurities and different housing materials." Journal of Power Sources 267:255-259, 2014, doi: 10.1016/j.jpowsour. 2014.05.080.
[6]Deng, Z., Lin, X., Huang, Z., Meng, J. et al., "Recent Progress on Advanced Imaging Techniques for LithiumвҖҗIon Batteries." Advanced Energy Materials 11 (2)2020, doi: 10.1002/aenm. 202000806.
[7]Rinkel, B. L. D., Hall, D. S., Temprano, I., and Grey, C. P., "Electrolyte Oxidation Pathways in Lithium-Ion Batteries." Journal of the American Chemical Society 142 (35):15058-15074, 2020, doi: 10.1021/jacs. 0c06363.
[8]Kong, W., Li, H., Huang, X., and Chen, L., "Gas evolution behaviors for several cathode materials in lithium-ion batteries." Journal of Power Sources 142 (1-2):285-291, 2005, doi: 10.1016/j.jpowsour. 2004. 10.008.
[9]Larsson, F., Andersson, P., Blomqvist, P., and Mellander, B. E., "Toxic fluoride gas emissions from lithium-ion battery fires." Sci Rep 7 (1):10018, 2017, doi: 10.1038/s41598-017-09784-z.
[10]Laszczynski, N., Solchenbach, S., Gasteiger, H. A., and Lucht, B. L., "Understanding Electrolyte Decomposition of Graphite/NCM811 Cells at Elevated Operating Voltage." Journal of The Electrochemical Society 166 (10):A1853-A1859, 2019, doi: 10.1149/2.0571910jes.
[11]Kim, S., Kim, H. S., Kim, B., Kim, Y. J. et al., "In Situ Gas Analysis by Differential Electrochemical Mass Spectrometry for Advanced Rechargeable Batteries: A Review." Advanced Energy Materials 2023, doi: 10.1002/aenm.202301983.
[12]Zhang, H., Chen, J., Zhang, B., Wu, X. et al., "Tracking gassing behavior in pouch cell by operando on-line electrochemical mass spectrometry." Journal of Energy Chemistry 84:286-291, 2023, doi: 10.1016/j.jechem. 2023.04.044.
[13]Li, H., Guo, S., and Zhou, H., "In-situ/operando characterization techniques in lithium-ion batteries and beyond." Journal of Energy Chemistry 59:191-211, 2021, doi: 10.1016/j.jechem.2020.11.020.
[14]Stancovski, V. and Badilescu, S., "In situ Raman spectroscopicвҖ“electrochemical studies of lithium-ion battery materials: a historical overview." Journal of Applied Electrochemistry 44 (1):23-43, 2013, doi: 10.1007/s10800-013-0628-0.
[15]Cai, T., Valecha, P., Tran, V., Engle, B. et al., "Detection of Li-ion battery failure and venting with Carbon Dioxide sensors." eTransportation 72021, doi: 10.1016/j. etran. 2020.100100.
[16]Xin, Y., Liu, C., Li, N., Lyu, S. et al., "In-situ monitoring of multiple signals evolution behaviour for commercial lithium-ion batteries during internal short circuit." Applied Energy 3502023, doi: 10.1016/j.apenergy.2023. 121754.
[17]Galushkin, N. Р•., Yazvinskaya, N. N., and Galushkin, D. N., "Mechanism of Gases Generation during Lithium-Ion Batteries Cycling." Journal of The Electrochemical Society 166 (6):A897-A908, 2019, doi: 10.1149/2.0041906jes.
[18]Gerelt-Od, B., Kim, J., Shin, E., Kang, H. et al., "In situ Raman investigation of resting thermal effects on gas emission in charged commercial 18650 lithium ion batteries." Journal of Industrial and Engineering Chemistry 96:339-344, 2021, doi: 10.1016/ j.jiec.2021.01.039.
[19]Lyu, S., Li, N., Sun, L., Jiao, S. et al., "Rapid operando gas monitor for commercial lithium ion batteries: Gas evolution and relation with electrode materials." Journal of Energy Chemistry 72:14-25, 2022, doi: 10.1016/j.jechem. 2022.04.010.
[20]Wang, S., Liu, J., Rafiz, K., Jin, Y. et al., "An On-Line Transient Study on Gassing Mechanism of Lithium Titanate Batteries." Journal of The Electrochemical Society 166 (16):A4150-A4157, 2019, doi: 10.1149/ 2.0631916jes.
[21]Schmiegel, J.-P., LeiГҹing, M., Weddeling, F., Horsthemke, F. et al., "Novel In Situ Gas Formation Analysis Technique Using a Multilayer Pouch Bag Lithium Ion Cell Equipped with Gas Sampling Port." Journal of The Electrochemical Society 167 (6)2020, doi: 10.1149/1945-7111/ab8409.
